Microscopes let us peek into worlds the naked eye can’t resolve, from the ridges of a butterfly wing to the lattice of a crystal and the organelles inside living cells. If you’ve ever wondered which microscope does what (and why there are so many types), this guide walks you through the types of microscope landscape step by step, mixing plain-English explanations with practical tips so you can pick the right tool with confidence.
Why Microscopes Matter
Microscopes expand our vision in two ways: they enlarge tiny features (magnification) and separate details that are very close together (resolution). Magnification without resolution is like zooming in on a blurry photo, you get a bigger blur. The best microscope for your job is the one that delivers enough resolution, contrast, and field of view for your specific sample and question.
Magnification vs. Resolution
- Magnification is how large the image appears relative to the object.
- Resolution is the smallest distance between two points that you can distinguish as separate.
In light microscopy, resolution is limited by the wavelength of light and lens numerical aperture (NA). Electron and scanning probe microscopes use electrons or physical probes to push resolution far below the optical limit.
How Contrast Is Created
Most biological samples are transparent. You get contrast by:
- Absorption/Staining (dyes or fluorescence)
- Phase Manipulation (phase-contrast, DIC)
- Scattering (dark-field)
- Fluorescence Emission (fluorophores lighting up target structures)
Major Categories at a Glance
- Light (Optical) Microscopes: Work with visible (or near-visible) light; great for live cells, tissues, and everyday tasks.
- Electron Microscopes: Use electrons for nanometer-scale details; require vacuum and special prep.
- Scanning Probe Microscopes: Use a physical probe to feel surfaces at the atomic scale.
- Other Specialized Modalities: Tailored to metals, thick tissues, crystals, or nondestructive 3D imaging.
Light (Optical) Microscopes
Compound Light Microscope

What it is: The classic lab microscope with multiple objective lenses (e.g., 4×, 10×, 40×, 100× oil) and eyepieces. It uses transmitted light to view thin, mounted specimens on slides.
Best for: Stained tissues, smears, prepared slides, and microorganisms.
Strengths:
- Affordable entry points
- Easy to use and maintain
- Works with many contrast methods (bright-field, phase-contrast, dark-field, fluorescence with add-ons)
Limits:
- Depth is shallow at high magnification
- Thick or opaque samples are hard to visualize without special techniques
Use Cases & Limits
- Blood smears, bacterial morphology, histology—this is the everyday workhorse.
- Not ideal for 3D structure; for that, stereo or confocal may fit better.
Key Specs to Check
- Numerical Aperture (NA): Higher NA → better resolution and brightness.
- Plan Objectives: “Plan” lenses flatten the field for edge-to-edge sharpness.
- Köhler Illumination: Proper setup yields uniform, high-contrast lighting.
- Stage & Focus: Smooth mechanical stage and fine focus make life easier.
Stereo (Dissecting) Microscope
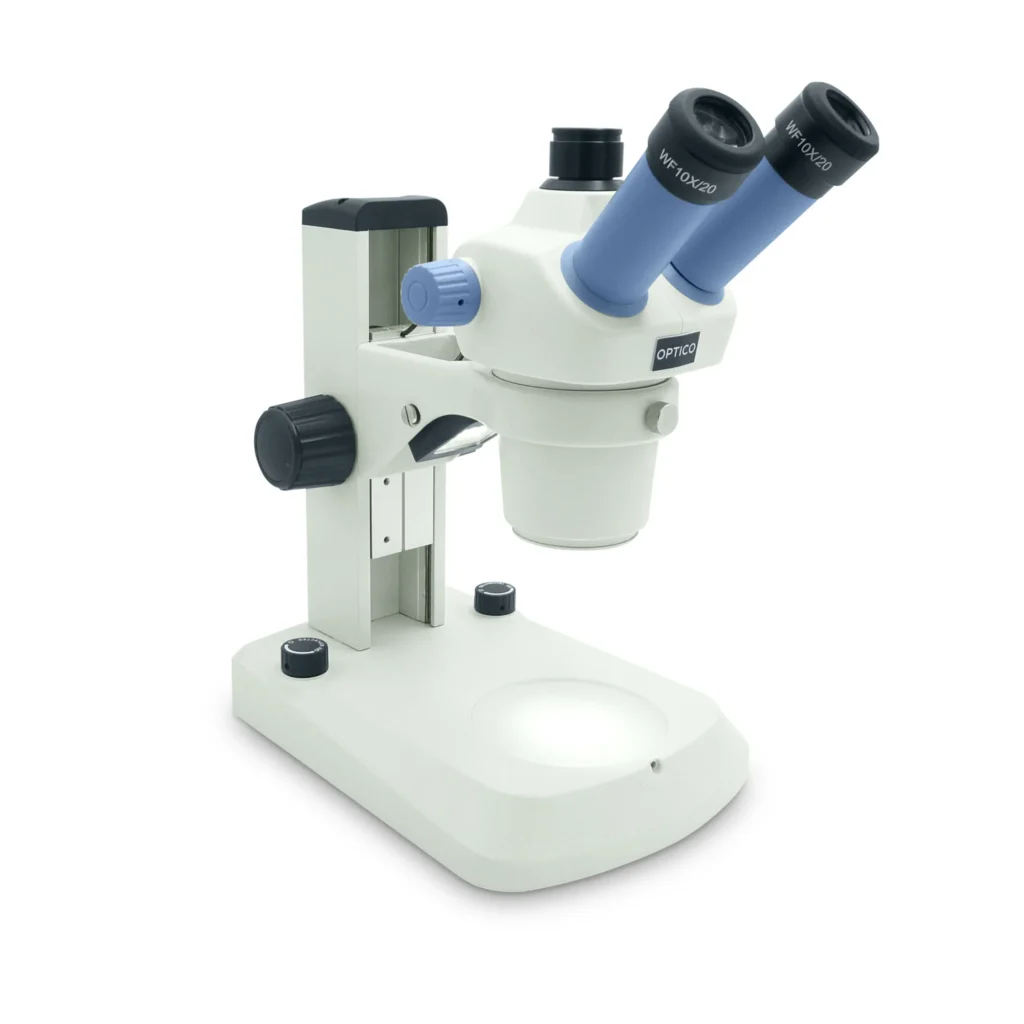
What it is: Low magnification (typically 5×–50×) with two optical paths for 3D viewing.
Best for: Dissections, insects, minerals, circuit boards, and quality control.
Strengths:
- Large working distance for tools
- Great depth perception
Limits:
- Not designed for high magnification or internal cell details
Phase-Contrast Microscope

What it is: Converts subtle phase shifts in transparent specimens into intensity differences.
Best for: Live, unstained cells, think cell culture, protozoa, sperm analysis.
Strengths:
- Non-destructive, natural-state imaging
- Fast and simple once aligned
Limits:
- Halos around specimens; not great for thick tissues
Differential Interference Contrast (DIC/Nomarski)
What it is: Uses polarized light and prisms to produce images with shadowed, relief-like contrast.
Best for: Fine structures in transparent samples—organelles, membranes.
Strengths:
- Crisp, high-contrast images with minimal halos
- Excellent for live-cell dynamics
Limits:
- More expensive optics; requires precise polarization
Dark-Field Microscope

What it is: Illuminates the sample so only scattered light enters the objective; the field appears dark.
Best for: Tiny, low-contrast objects like thin bacteria, flagella, nanoparticles.
Strengths:
- Dramatic contrast without staining
Limits:
- Sensitive to dust/contamination; limited quantitative info
Polarizing Microscope

What it is: Uses polarized light and analyzers to reveal birefringence.
Best for: Minerals, polymers, fibers, crystalline structures.
Strengths:
- Identifies crystal orientation, strain, and composition clues
Limits:
- Requires understanding of polarization techniques
Fluorescence Microscope
What it is: Excites fluorophores and collects emitted light through filters.
Best for: Target-specific labeling—DNA, proteins, organelles. Widely used in diagnostics and research.
Strengths:
- High specificity and sensitivity
- Multi-channel imaging for complex labeling
Limits:
- Photobleaching and phototoxicity
- Requires proper controls and filters
Confocal Laser Scanning Microscope
What it is: Uses a pinhole to reject out-of-focus light, scanning point-by-point to build images.
Best for: Thick specimens, optical sectioning, 3D reconstructions, colocalization.
Strengths:
- Sharper images in z-stacks
- Great for tissues and organoids
Limits:
- Slower and more expensive than widefield
- Increased light dose to samples
Super-Resolution Light Microscopy (STED, PALM/STORM, SIM)
What it is: Techniques that beat the diffraction limit of light, reaching tens of nanometers in resolution.
Best for: Nanoscale protein organization, synapses, cytoskeleton, membrane domains.
Strengths:
- Resolves structures conventional optics can’t
Limits:
- Complex instrumentation and sample prep
- Often slower and more light-intensive
Electron Microscopes
Transmission Electron Microscope (TEM)

What it is: Electrons pass through ultra-thin specimens. Magnifications can exceed 1,000,000× with nanometer to sub-nanometer resolution.
Best for: Ultrastructure of cells, viruses, nanomaterials, and crystallography.
Strengths:
- Unmatched internal detail
- Diffraction and tomography options for structure analysis
Limits:
- Demanding sample prep (ultrathin sectioning, staining)
- Vacuum environment; not for live samples
Scanning Electron Microscope (SEM)

What it is: Scans a focused electron beam over the surface; detectors collect emitted signals to map topography and composition.
Best for: Surface structures—microfabrication, insects, fracture surfaces, powders.
Strengths:
- Striking 3D-like images
- Large depth of field
- With EDS/EDX, you can get elemental analysis
Limits:
- Usually needs conductive coating (gold/carbon) for nonconductive samples
- Vacuum; sample dehydration required
Scanning Transmission Electron Microscope (STEM)
What it is: Combines SEM’s scanning with TEM’s transmission; excels at atomic-scale imaging and chemical mapping.
Best for: Nanotechnology, semiconductors, catalyst particles.
Strengths:
- Spectroscopy (EELS/EDS) for composition and bonding
- Atomic-column resolution in advanced setups
Limits:
- High cost, advanced prep and operation
Scanning Probe Microscopes
Atomic Force Microscope (AFM)

What it is: A sharp tip on a cantilever scans the surface; deflection maps topography down to fractions of a nanometer.
Best for: Polymers, soft matter, biomolecules, nano-roughness.
Strengths:
- Works in air or liquid; can image live cells’ surfaces
- Measures mechanical properties (stiffness, adhesion)
Limits:
- Slow scan speed; small scan areas
- Tips can wear or be contaminated
Scanning Tunneling Microscope (STM)

What it is: Measures quantum tunneling current between a conductive tip and surface at angstrom distances.
Best for: Conductive/semiconductive surfaces; atomic lattices, adsorbates.
Strengths:
- True atomic resolution on suitable samples
- Manipulation of atoms/molecules is possible
Limits:
- Requires conductive samples and vibration isolation
Near-Field Scanning Optical Microscopy (NSOM/SNOM)
What it is: Brings a subwavelength aperture or tip extremely close to the sample to bypass diffraction.
Best for: Optical properties at the nanoscale—plasmonics, photonics.
Strengths:
- Optical signals with nanometric spatial resolution
Limits:
- Complex alignment; limited signal throughput
Other Specialized Microscopes
Inverted Microscopes
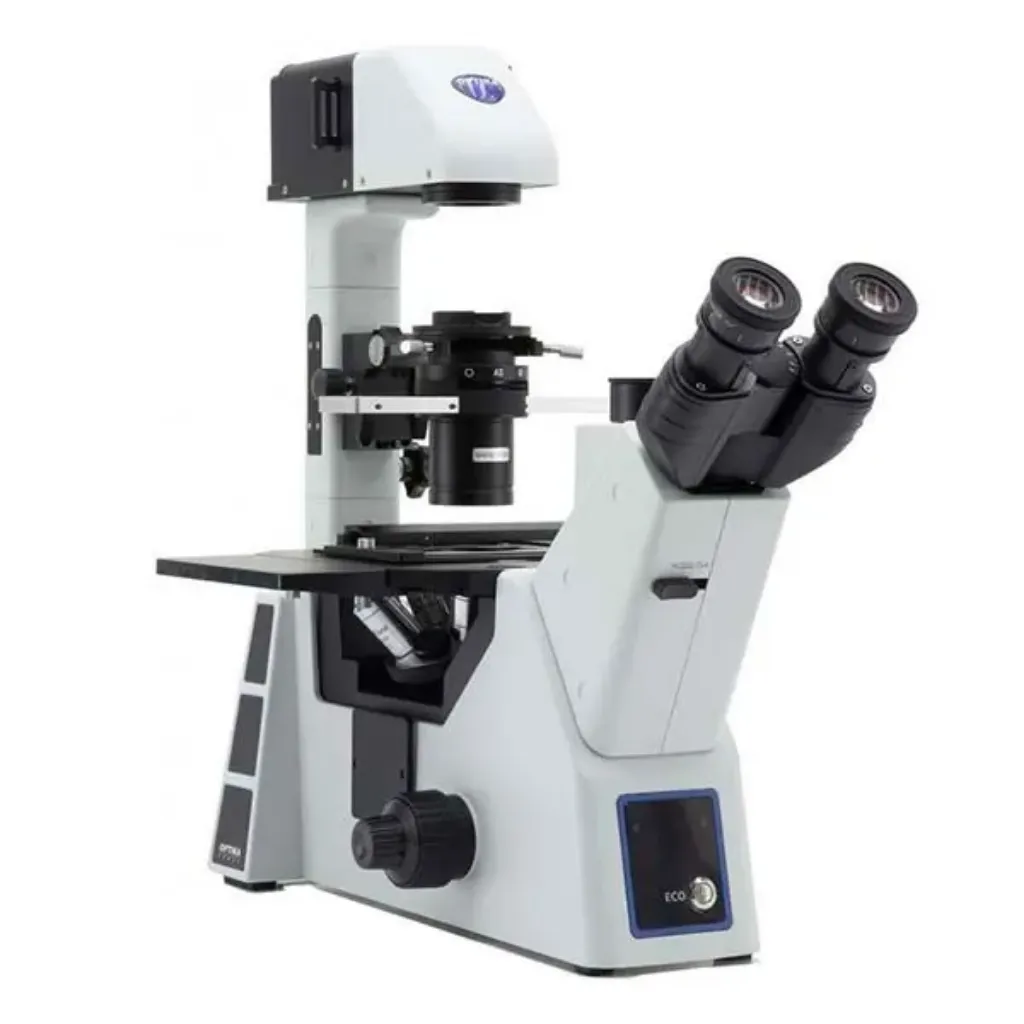
What it is: Objectives below the stage; condenser above. Designed for dishes/flasks.
Best for: Live-cell imaging in culture vessels, time-lapse studies.
Metallurgical Microscopes
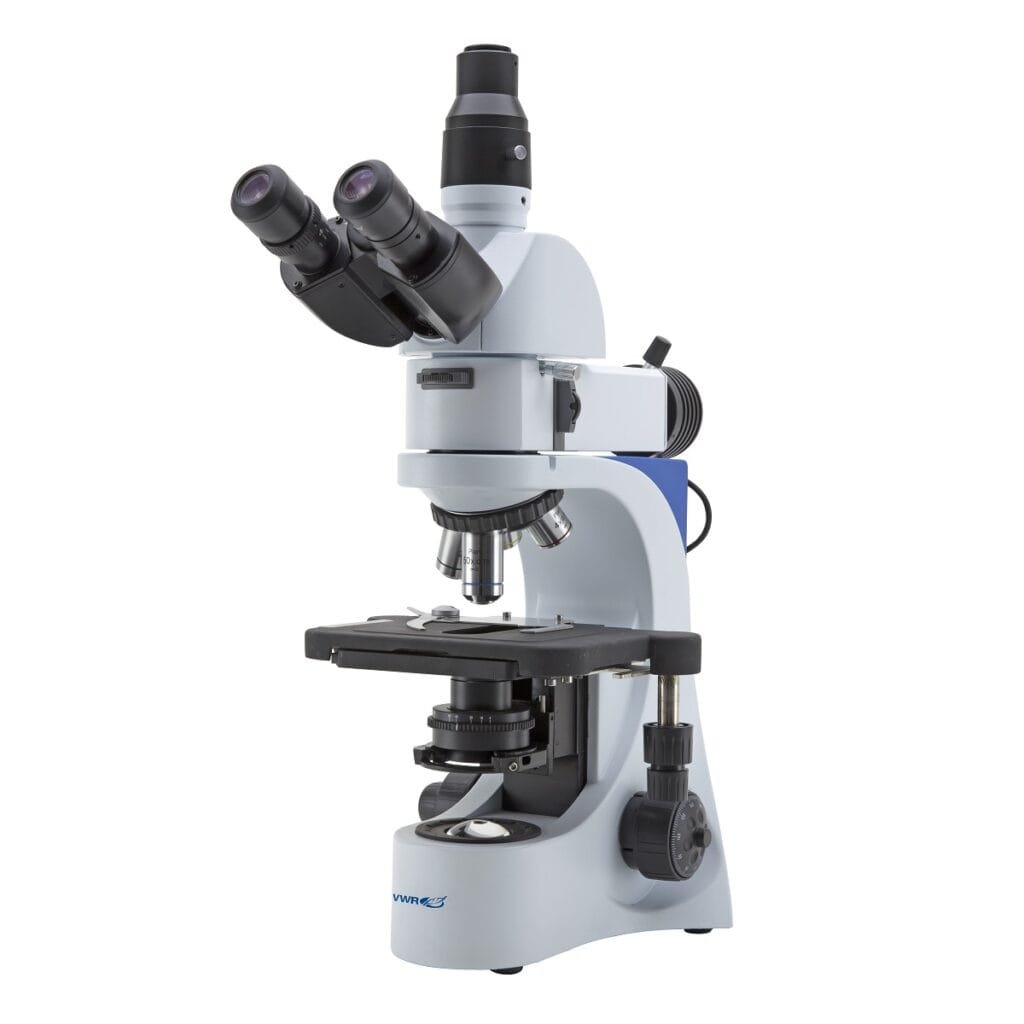
What it is: Uses reflected light to inspect opaque materials.
Best for: Metals, welds, microstructures, inclusions, failure analysis.
Confocal Variants: Spinning Disk & Multiphoton
- Spinning Disk: Parallelizes confocal pinholes for fast live imaging, lower light dose.
- Multiphoton (Two-Photon): Uses near-infrared to excite fluorescence deep in tissues with less photodamage—great for thick or living specimens.
X-Ray Microscopy & Micro-CT
What it is: Uses X-rays to penetrate samples and reconstruct 3D volumes.
Best for: Non-destructive 3D imaging of fossils, electronics, bones, composites.
Scanning Acoustic Microscopy
What it is: Uses high-frequency ultrasound to map elasticity and internal features.
Best for: Delamination in composites, microelectronics inspection, soft materials.
Ion & Helium Ion Microscopes
What it is: Focused ion beams (Ga⁺ or He⁺) for imaging and even nanofabrication (milling, cross-sectioning).
Best for: Extreme surface sensitivity, patterning, and preparing TEM lamellae.
Digital/USB Microscopes

What it is: Compact, camera-based devices for screens.
Best for: Education, quick inspections, hobby electronics, coins, stamps.
Pros: Affordable, easy sharing, macro to micro range.
Cons: Limited optics, lower resolution vs. research-grade systems.
How to Choose the Right Microscope
Match Modality to Sample & Goal
- Transparent biology: Start with compound + phase or DIC; add fluorescence if tagging is needed.
- 3D hand work: Choose a stereo microscope.
- Nanostructures/surfaces: SEM or AFM; internal nanoscale → TEM/STEM.
- Thick tissues/live imaging: Confocal (spinning disk for speed) or multiphoton.
Resolution, NA, and Working Distance
- NA drives resolution and brightness; higher NA means finer detail but shallower depth.
- Working distance matters if you need room for tools or thick samples (stereo, long-WD objectives).
Illumination & Optics Quality
- Seek LED illumination with adjustable intensity and set up Köhler illumination for even lighting.
- Plan apochromats (if budget allows) give top color correction and resolution.
Cameras, Software, and Data
- Match camera pixel size to objective NA (sampling theory) to avoid oversampling/undersampling.
- Look for easy white balance, exposure control, and scalable file formats (TIFF).
- Consider analysis plugins (cell counting, particle sizing, stitching, deconvolution).
Budget Tiers & Realistic Expectations
- Entry (<$500/low-cost): Student compound or USB digital; ideal for basic needs.
- Mid ($1k–$10k): Quality optics, phase/DIC, fluorescence, decent cameras.
- Pro (>$10k to $500k+): Confocal, multiphoton, SEM/TEM/AFM with advanced modules.
Sample Preparation Essentials
Fixation, Staining, and Mounting
- Fixation (e.g., formaldehyde, glutaraldehyde) locks structure in place.
- Staining (H&E, Gram, fluorescent dyes) adds contrast or specificity.
- Mounting media and clean coverslips prevent bubbles and preserve samples.
Coating & Sectioning for EM
- TEM: Dehydration, embedding, ultramicrotomy (50–100 nm sections), heavy-metal staining.
- SEM: Dehydrate and sputter-coat nonconductive samples with a thin metal layer.
Avoiding Common Prep Pitfalls
- Dust and fingerprints kill image quality—handle slides by the edges.
- Avoid thick mounts for transmitted light.
- Control photobleaching in fluorescence (antifade, minimal exposure).
Maintenance & Best Practices
Cleaning Optics the Right Way
- Use a blower first, then lens paper with minimal lens cleaner.
- Never scrub objectives; protect oil immersion lenses from dust.
Calibration, Köhler Illumination, and Care
- Calibrate scale bars with a stage micrometer.
- Align for Köhler to maximize contrast and uniformity.
- Keep instruments covered and in low-dust, stable environments.
Storage & Environment
- Dry cabinets help prevent fungus on optics in humid climates.
- Vibration isolation matters for high magnification and scanning probe methods.
Microscopy Across Fields
Biology & Medicine
- Diagnose pathogens (bright-field, fluorescence), track proteins (GFP), map neural circuits (confocal, multiphoton), and study ultrastructure (TEM).
Materials Science & Engineering
- Evaluate grain size, fractures, coatings (metallurgical, SEM), and atomic interfaces (STEM/AFM).
Forensics, Geology, and Education
- Fiber analysis, gunshot residue (SEM/EDS), mineral identification (polarized light), and student learning (digital and compound scopes).
Conclusion
Choosing among the many types of microscopes isn’t about chasing the highest magnification number—it’s about pairing the right contrast, resolution, and workflow to your sample and question. For live, transparent cells, phase-contrast or DIC makes magic without stains. For pinpoint targeting of molecules, fluorescence (and confocal for thickness) is king. For surfaces and nanoscale detail, SEM, TEM, STEM, and AFM open doors that light alone cannot. With a clear goal, a basic grasp of contrast and resolution, and attention to sample prep and maintenance, you’ll consistently get images that answer real scientific or practical questions—beautifully and reliably.
FAQs
1) What’s the difference between stereomicroscopes and compound microscopes?
Stereo microscopes give low magnification with a 3D view and long working distance—perfect for dissection and inspection. Compound microscopes offer higher magnification for thin, transmitted-light specimens like cells and tissues.
2) Do I need fluorescence, or will bright-field be enough?
If your targets are naturally visible with stains (e.g., H&E histology), bright-field may suffice. If you need to see specific molecules or structures (proteins, DNA, organelles) with high specificity, go for fluorescence.
3) Why are electron microscopes so expensive and complex?
They require vacuum systems, high-voltage electron sources, stable mechanics, and specialized sample prep. In return, they deliver nanometer to atomic-scale detail that optical systems can’t reach.
4) Can I image live cells with confocal or super-resolution?
Yes, but manage phototoxicity and photobleaching. Spinning disk confocal and gentle illumination help; super-resolution often needs brighter light and careful optimization for live samples.
5) What does numerical aperture (NA) really buy me?
Higher NA improves resolution and brightness, letting you resolve finer details. It typically reduces depth of field, so careful focusing and thinner samples become more important.

0 Comments